Hofmann elimination See also References External links Navigation menu"Researches into the molecular constitution of the organic bases""Beiträge zur Kenntniss der flüchtigen organischen Basen"10.1002/jlac.18510780302"trans-Cyclooctene"10.1002/cber.18940270163An animation of the mechanism of the Hofmann elimination
Elimination reactionsOlefination reactionsName reactions
quaternary ammoniumalkenemethyl iodidesilver oxidewaterquaternaryammonium iodidehydroxylelimination reactionZaitsev's ruleAugust Wilhelm von Hofmanntrans-cyclooctenechemical testhydrogen iodideiodomethane
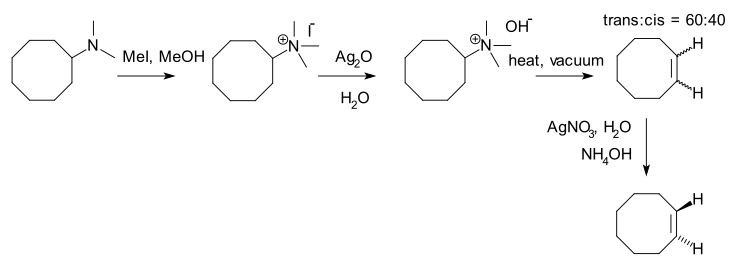
Hofmann elimination, also known as exhaustive methylation, is a process where a quaternary ammonium reacts to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.
After the first step, a quaternary ammonium iodide salt is created. After replacement of iodine by a hydroxyl anion, an elimination reaction takes place to form the alkene.
With asymmetrical amines, the major alkene product is the least substituted and generally the least stable, an observation known as the Hofmann rule. This is in direct contrast to normal elimination reactions where the more substituted, stable product is dominant (Zaitsev's rule).
The reaction is named after its discoverer, August Wilhelm von Hofmann.[1][2]
An example is the synthesis of trans-cyclooctene:[3]
- Hoffmann reaction and cope reaction
In a related chemical test called Herzig–Meyer alkimide group determination a tertiary amine with at least one methyl group and lacking a beta-proton is allowed to react with hydrogen iodide to the quaternary ammonium salt which when heated degrades to iodomethane and the secondary amine.[4]
See also
- Hofmann rearrangement
- Emde degradation
References
^ Hofmann, A. W. (1851). "Researches into the molecular constitution of the organic bases". Philosophical Transactions of the Royal Society of London. 141: 357–398..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Aug. Wilh. von Hofmann (1851). "Beiträge zur Kenntniss der flüchtigen organischen Basen" [Contribution to [our] knowledge of volatile organic bases]. Annalen der Chemie und Pharmacie (in German). 78 (3): 253–286. doi:10.1002/jlac.18510780302.
^ Arthur C. Cope; Robert D. Bach (1973). "trans-Cyclooctene". Organic Syntheses.; Collective Volume, 5, p. 315
^ J. Herzig; H. Meyer (1894). "Ueber den Nachweis und die Bestimmung des am Stickstoff gebundenen Alkyls". Berichte der deutschen chemischen Gesellschaft. 27 (1): 319–320. doi:10.1002/cber.18940270163.
External links
- An animation of the mechanism of the Hofmann elimination
Elimination reactions, Name reactions, Olefination reactionsUncategorized

