Indirubin Research References Navigation menu479-41-4Interactive imageCHEBI:92322ChEMBL1276127ChEMBL31857834477010DB12379100.119.64610177V86L8P74GI"Indirubin""Indirubins Decrease Glioma Invasion by Blocking Migratory Phenotypes in Both the Tumor and Stromal Endothelial Cell Compartments"10.1158/0008-5472.CAN-10-30260008-5472428848021697283"Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis"10.3748/wjg.v19.i17.271836453932367488210.1002/ijc.259091097-021521207415e
KetonesLactams
chemical compoundpurple urine bag syndromeindoxyl sulfatePLK1PIN1CDC25B genesmall-cell lung cancerglioblastomachronic myeloid leukemiaulcerative colitisanti-inflammatoryanti-angiogenesis
 | |
| Names | |
|---|---|
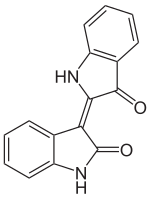
IUPAC name (3Z)-3-(3-Oxo-1,3-dihydro-2H-indol-2-ylidene)-1,3-dihydro-2H-indol-2-one | |
| Other names Indigo red | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.119.646 |
EC Number | 610-392-0 |
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C16H10N2O2 |
Molar mass | 7002262267999999999♠262.268 g·mol−1 |
| Hazards | |
GHS pictograms |  |
GHS signal word | Warning |
GHS hazard statements | H315, H319, H335 |
GHS precautionary statements | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Indirubin is a chemical compound most often produced as a byproduct of bacterial metabolism. For instance, it is one of the compounds responsible for the generally benign condition purple urine bag syndrome, resulting from bacteria metabolizing indoxyl sulfate found naturally in urine.
Indirubin is a chemical constituent of indigo naturalis (also known as qing dai), which has been used for hundreds of years in traditional Chinese medicine. It is produced by collecting the waste products from the bacterial degradation of specific forms of vegetation.
Research
Indirubin exerts its effects on the human body by downregulating expression of genes. Genes PLK1 and PIN1, both oncogenic, have been shown to be affected by indirubin. Indirubin has, in vitro and in vivo, been shown to reduce expression of the CDC25B gene, which codes for production of CDC25B enzyme. CDC stands for cell-division-cycle, and is used in cellular reproduction. Studies suggest that mouse cells are viable after the CDC25B (and CDC25C) genes are "knocked out", but removal of CDC25A results in non-viable cells.
Indirubin has not been shown to prevent or treat cancer in humans.[1] However, it is being studied for treatment of small-cell lung cancer, glioblastoma,[2] and chronic myeloid leukemia, either alone or in conjunction with more typical cancer management treatments. It has also been studied for potential use in the treatment of ulcerative colitis, an immune-modulated disease process.[3]
Indirubin shows anti-inflammatory and anti-angiogenesis properties in vitro.[4]
References
^ "Indirubin". Memorial Sloan Kettering Cancer Center..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Williams, Shanté P.; Nowicki, Michal O.; Liu, Fang; Press, Rachael; Godlewski, Jakub; Abdel-Rasoul, Mahmoud; Kaur, Balveen; Fernandez, Soledad A.; Chiocca, E. Antonio (2011-08-15). "Indirubins Decrease Glioma Invasion by Blocking Migratory Phenotypes in Both the Tumor and Stromal Endothelial Cell Compartments". Cancer Research. 71 (16): 5374–5380. doi:10.1158/0008-5472.CAN-10-3026. ISSN 0008-5472. PMC 4288480. PMID 21697283.
^ Hideo Suzuki; Tsuyoshi Kaneko; Yuji Mizokami; Toshiaki Narasaka; Shinji Endo; Hirofumi Matsui; Akinori Yanaka; Aki Hirayama; Ichinosuke Hyodo (2013). "Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis". World J Gastroenterol. 19 (17): 2718–2722. doi:10.3748/wjg.v19.i17.2718. PMC 3645393. PMID 23674882.
^ Zhang, Xiaoli; Song, Yajuan; Wu, Yuanyuan; Dong, Yanmin; Lai, Li; Zhang, Jing; Lu, Binbin; Dai, Fujun; He, Lijun (2011-11-15). "Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell". International Journal of Cancer. 129 (10): 2502–2511. doi:10.1002/ijc.25909. ISSN 1097-0215. PMID 21207415.
Ketones, LactamsUncategorized