PARK7 Contents Structure Function Clinical significance Interactions See also References Further reading Navigation menuPDBeRCSB1J421P5F1PDV1PDW1PE01Q2U1SOA1UCF2OR32R1T2R1U2R1V2RK32RK42RK63B363B383B3A3BWE3CY63CYF3CZ93CZA3EZG3F713SF84BTE4MNT4MTC4N0M4N124OGF4OQ44P2G4P344P354P364ZGG4RKW4RKY4S0ZPARK7213563738295PARK7More reference expression datareceptor bindingrepressing transcription factor bindingcytokine bindingcore promoter bindingcupric ion bindingtranscription factor bindingL-dopa decarboxylase activator activitypeptidase activitykinase bindingtyrosine 3-monooxygenase activator activityenzyme bindingmRNA bindingprotein homodimerization activityscaffold protein bindingsmall protein activating enzyme bindingsingle-stranded DNA bindingubiquitin-like protein conjugating enzyme bindingprotein bindingperoxiredoxin activitydouble-stranded DNA bindingcopper ion bindingmercury ion bindingtranscription coactivator activityoxidoreductase activity, acting on peroxide as acceptorandrogen receptor bindingsuperoxide dismutase copper chaperone activityRNA bindingidentical protein bindingubiquitin-specific protease bindinghydrolase activitycuprous ion bindingcadherin bindingprotein deglycase activitycytoplasmcytosolmembranemitochondrial intermembrane spacemitochondrionneuron projectioncell nucleusmitochondrial respiratory chain complex Iendoplasmic reticulummembrane raftchromatinextracellular exosomeplasma membranemitochondrial matrixcell bodyPML bodypresynapseperinuclear region of cytoplasmcell-cell adherens junctionaxonnucleoplasmnegative regulation of neuron apoptotic processpositive regulation of transcription regulatory region DNA bindingnegative regulation of protein sumoylationnegative regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathwaynegative regulation of protein bindingsynaptic transmission, dopaminergicpositive regulation of interleukin-8 productionnegative regulation of proteasomal ubiquitin-dependent protein catabolic processRas protein signal transductioncellular response to glyoxalnegative regulation of protein acetylationpositive regulation of reactive oxygen species biosynthetic processpositive regulation of oxidative phosphorylation uncoupler activitypositive regulation of protein kinase B signalingnegative regulation of protein catabolic processadult locomotory behaviorregulation of neuron apoptotic processnegative regulation of extrinsic apoptotic signaling pathwaynegative regulation of cell deathglycolate biosynthetic processpositive regulation of protein homodimerization activitynegative regulation of gene expressionsingle fertilizationpositive regulation of peptidyl-serine phosphorylationpositive regulation of mitochondrial electron transport, NADH to ubiquinonenegative regulation of hydrogen peroxide-induced neuron intrinsic apoptotic signaling pathwaynegative regulation of oxidative stress-induced cell deathinflammatory responsenegative regulation of oxidative stress-induced neuron intrinsic apoptotic signaling pathwayregulation of supramolecular fiber organizationnegative regulation of neuron deathnegative regulation of protein phosphorylationnegative regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathwaynegative regulation of protein kinase activitynegative regulation of oxidative stress-induced neuron deathmembrane depolarizationprotein stabilizationmitochondrion organizationpositive regulation of sequence-specific DNA binding transcription factor activitynegative regulation of death-inducing signaling complex assemblynegative regulation of apoptotic processproteolysisnegative regulation of protein K48-linked deubiquitinationenzyme active site formation via L-cysteine sulfinic aciddopamine uptake involved in synaptic transmissionpositive regulation of tyrosine 3-monooxygenase activitypositive regulation of L-dopa decarboxylase activitynegative regulation of hydrogen peroxide-induced neuron deathregulation of inflammatory responseregulation of androgen receptor signaling pathwaynegative regulation of hydrogen peroxide-induced cell deathpositive regulation of autophagy of mitochondrionresponse to hydrogen peroxidedetoxification of copper ionpositive regulation of superoxide dismutase activitypositive regulation of dopamine biosynthetic processpositive regulation of protein localization to nucleuspositive regulation of L-dopa biosynthetic processactivation of protein kinase B activitymembrane hyperpolarizationdetoxification of mercury ionnegative regulation of TRAIL-activated apoptotic signaling pathwaycellular oxidant detoxificationnegative regulation of ubiquitin-specific protease activityregulation of TRAIL receptor biosynthetic processautophagycellular response to reactive oxygen speciesregulation of mitochondrial membrane potentialnegative regulation of protein ubiquitinationhydrogen peroxide metabolic processpositive regulation of gene expressionnegative regulation of protein export from nucleusnegative regulation of ubiquitin-protein transferase activitypositive regulation of oxidative stress-induced intrinsic apoptotic signaling pathwaycellular response to oxidative stresspositive regulation of androgen receptor activitypositive regulation of pyrroline-5-carboxylate reductase activitypositive regulation of transcription from RNA polymerase II promotercellular response to hydrogen peroxidemethylglyoxal metabolic processlactate biosynthetic processpeptidyl-cysteine deglycationpeptidyl-arginine deglycationpeptidyl-lysine deglycationprotein deglycation, glyoxal removalprotein deglycation, methylglyoxal removalglutathione deglycationglyoxal metabolic processnegative regulation of nitrosative stress-induced intrinsic apoptotic signaling pathwaypositive regulation of acute inflammatory response to antigenic stimulusprotein deglycosylationpositive regulation of NAD(P)H oxidase activityDNA repaircellular response to DNA damage stimulusinsulin secretionglucose homeostasisguanine deglycationguanine deglycation, methylglyoxal removalguanine deglycation, glyoxal removalnegative regulation of reactive oxygen species biosynthetic processAmigoQuickGO1131557320ENSG00000116288ENSMUSG00000028964Q99497Q99LX0NM_001123377NM_007262NM_020569NP_001116849NP_009193NP_065594Chr 1: 7.95 – 7.99 MbChr 4: 150.9 – 150.91 MbGRCh38: Ensembl release 89: ENSG00000116288GRCm38: Ensembl release 89: ENSMUSG00000028964"Human PubMed Reference:""Mouse PubMed Reference:""Entrez Gene: PARK7""Uniprot: Q99497 - PARK7_HUMAN"10.1074/jbc.M3058782001279648210.1074/jbc.M3042212001276121410.1074/jbc.M30451720012939276"DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation"10.1371/journal.pbio.00203625211771550287410.1016/j.jmb.2005.12.0301640351910.1248/bpb.31.132118591768"Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity"10.1074/jbc.M113.48209138293652379295710.1126/science.107720912446870"Neuron-specific protein interactions of Drosophila CASK-β are revealed by mass spectrometry"10.3389/fnmol.2014.000584075472250714381261205310.1074/jbc.M1017302001147707010.1016/S0896-6273(02)01166-21252676710.1007/s00109-003-0512-11471235110.1016/j.coph.2003.09.0051501884310.1007/s00441-004-0922-615503154"Genetics of Parkinson disease"10.1602/neurorx.1.2.2355349351571702410.1007/978-3-211-45295-0_331701753210.1385/JMN:29:3:2151708578010.1006/bbrc.1997.6132907031010.1016/S0378-1119(00)00590-411223268"Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36"10.1086/32299612354911146217410.1074/jbc.M1017302001147707010.1126/science.10772091244687010.1007/s100720200069125483431261205310.1074/jbc.M3042212001276121410.1074/jbc.M3058782001279648210.1002/mds.104221281565310.1074/jbc.M30427220012851414e
Genes on human chromosome 1
1131557320ENSG00000116288ENSMUSG00000028964Q99497Q99LX0proteingeneβ-strandsα-helicesdimerpeptidaseoxidative stressandrogen receptorPyrroloquinoline quinoneParkinson's diseasecoppermercuryParkinson's diseaseinteract
| PARK7 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | PARK7, DJ-1, DJ1, HEL-S-67p, Parkinsonism associated deglycase, GATD2 | ||||||||||||||||||||||||
| External IDs | MGI: 2135637 HomoloGene: 38295 GeneCards: PARK7 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez |
|
| |||||||||||||||||||||||
| Ensembl |
|
| |||||||||||||||||||||||
| UniProt |
|
| |||||||||||||||||||||||
| RefSeq (mRNA) |
|
| |||||||||||||||||||||||
| RefSeq (protein) |
|
| |||||||||||||||||||||||
| Location (UCSC) | Chr 1: 7.95 – 7.99 Mb | Chr 4: 150.9 – 150.91 Mb | |||||||||||||||||||||||
PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Protein deglycase DJ-1, also known as Parkinson disease protein 7, is a protein which in humans is encoded by the PARK7 gene.[5]
Contents
1 Structure
1.1 Gene
1.2 Protein
2 Function
3 Clinical significance
4 Interactions
5 See also
6 References
7 Further reading
Structure
Gene
The gene PARK7, also known as DJ-1, encodes a protein of the peptidase C56 family. The human gene PARK7 has 8 Exons and locates at chromosome band 1p36.23.[5]
Protein
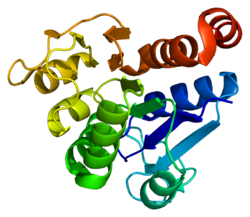
The human protein deglycase DJ-1 is 20 kDa in size and composed of 189 amino acids with seven β-strands and nine α-helices in total and is present as a dimer.[6][7][8] It belongs to the peptidase C56 family of proteins.
The protein structures of human protein DJ-1, Escherichia coli chaperone Hsp31, YhbO and YajL and an Archaea protease are evolutionarily conserved.[9]
Function
Under an oxidative condition, protein deglycase DJ-1 inhibits the aggregation of α-synuclein via its chaperone activity,[10][11] thus functions as a redox-sensitive chaperone and as a sensor for oxidative stress. Accordingly, DJ-1 apparently protects neurons against oxidative stress and cell death.[5] In parallel, Protein DJ-1 acts as a positive regulator of androgen receptor-dependent transcription.
Pyrroloquinoline quinone (PQQ) has been shown to reduce the self-oxidation of the DJ-1 protein, an early step in the onset of some forms of Parkinson's disease.[12]
Functional DJ-1 protein has been shown to bind metals and protect against metal-induced cytotoxicity from copper and mercury.[13]
Clinical significance
Defects in this gene are the cause of autosomal recessive early-onset Parkinson's disease 7.[5][14]
Interactions
PARK7 has been shown to interact with:
CASK,[15]
EFCAB6,[16] and
PIAS2.[17]
See also
- Parkinson's disease
References
^ abc GRCh38: Ensembl release 89: ENSG00000116288 - Ensembl, May 2017
^ abc GRCm38: Ensembl release 89: ENSMUSG00000028964 - Ensembl, May 2017
^ "Human PubMed Reference:"..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ "Mouse PubMed Reference:".
^ abcd "Entrez Gene: PARK7".
^ "Uniprot: Q99497 - PARK7_HUMAN".
^ Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H, Inagaki F (Aug 2003). "The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease". The Journal of Biological Chemistry. 278 (33): 31380–4. doi:10.1074/jbc.M305878200. PMID 12796482.
^ Tao X, Tong L (Aug 2003). "Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease". The Journal of Biological Chemistry. 278 (33): 31372–9. doi:10.1074/jbc.M304221200. PMID 12761214.
^ Lee SJ, Kim SJ, Kim IK, Ko J, Jeong CS, Kim GH, Park C, Kang SO, Suh PG, Lee HS, Cha SS (Nov 2003). "Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain". The Journal of Biological Chemistry. 278 (45): 44552–9. doi:10.1074/jbc.M304517200. PMID 12939276.
^ Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A (Nov 2004). "DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation". PLoS Biology. 2 (11): e362. doi:10.1371/journal.pbio.0020362. PMC 521177. PMID 15502874.
^ Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL (Mar 2006). "The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein". Journal of Molecular Biology. 356 (4): 1036–48. doi:10.1016/j.jmb.2005.12.030. PMID 16403519.
^ Nunome K, Miyazaki S, Nakano M, Iguchi-Ariga S, Ariga H (Jul 2008). "Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1". Biological & Pharmaceutical Bulletin. 31 (7): 1321–6. doi:10.1248/bpb.31.1321. PMID 18591768.
^ Björkblom B, Adilbayeva A, Maple-Grødem J, Piston D, Ökvist M, Xu XM, Brede C, Larsen JP, Møller SG (2013). "Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity". Journal of Biological Chemistry. 288 (31): 22809–20. doi:10.1074/jbc.M113.482091. PMC 3829365. PMID 23792957.
^ Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (Jan 2003). "Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism". Science. 299 (5604): 256–9. doi:10.1126/science.1077209. PMID 12446870.
^ Mukherjee K, Slawson JB, Christmann BL, Griffith LC (2014). "Neuron-specific protein interactions of Drosophila CASK-β are revealed by mass spectrometry". Frontiers in Molecular Neuroscience. 7: 58. doi:10.3389/fnmol.2014.00058. PMC 4075472. PMID 25071438.
^ Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SM, Ariga H (Feb 2003). "DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex". Molecular Cancer Research. 1 (4): 247–61. PMID 12612053.
^ Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H (Oct 2001). "DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor". The Journal of Biological Chemistry. 276 (40): 37556–63. doi:10.1074/jbc.M101730200. PMID 11477070.
Further reading
.mw-parser-output .refbeginfont-size:90%;margin-bottom:0.5em.mw-parser-output .refbegin-hanging-indents>ullist-style-type:none;margin-left:0.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>ddmargin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none.mw-parser-output .refbegin-100font-size:100%
Cookson MR (Jan 2003). "Pathways to Parkinsonism". Neuron. 37 (1): 7–10. doi:10.1016/S0896-6273(02)01166-2. PMID 12526767.
Bonifati V, Oostra BA, Heutink P (Mar 2004). "Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson's disease". Journal of Molecular Medicine. 82 (3): 163–74. doi:10.1007/s00109-003-0512-1. PMID 14712351.
Le W, Appel SH (Feb 2004). "Mutant genes responsible for Parkinson's disease". Current Opinion in Pharmacology. 4 (1): 79–84. doi:10.1016/j.coph.2003.09.005. PMID 15018843.
Abou-Sleiman PM, Healy DG, Wood NW (Oct 2004). "Causes of Parkinson's disease: genetics of DJ-1". Cell and Tissue Research. 318 (1): 185–8. doi:10.1007/s00441-004-0922-6. PMID 15503154.
Pankratz N, Foroud T (Apr 2004). "Genetics of Parkinson disease". NeuroRx. 1 (2): 235–42. doi:10.1602/neurorx.1.2.235. PMC 534935. PMID 15717024.
Heutink P (2006). "PINK-1 and DJ-1--new genes for autosomal recessive Parkinson's disease". Journal of Neural Transmission. Supplementum. Journal of Neural Transmission. Supplementa. 70 (70): 215–9. doi:10.1007/978-3-211-45295-0_33. ISBN 978-3-211-28927-3. PMID 17017532.
Lev N, Roncevic D, Roncevich D, Ickowicz D, Melamed E, Offen D (2007). "Role of DJ-1 in Parkinson's disease". Journal of Molecular Neuroscience. 29 (3): 215–25. doi:10.1385/JMN:29:3:215. PMID 17085780.
Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H (Feb 1997). "DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras". Biochemical and Biophysical Research Communications. 231 (2): 509–13. doi:10.1006/bbrc.1997.6132. PMID 9070310.
Taira T, Takahashi K, Kitagawa R, Iguchi-Ariga SM, Ariga H (Jan 2001). "Molecular cloning of human and mouse DJ-1 genes and identification of Sp1-dependent activation of the human DJ-1 promoter". Gene. 263 (1–2): 285–92. doi:10.1016/S0378-1119(00)00590-4. PMID 11223268.
van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, Snijders PJ, Testers L, Breedveld GJ, Horstink M, Sandkuijl LA, van Swieten JC, Oostra BA, Heutink P (Sep 2001). "Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36". American Journal of Human Genetics. 69 (3): 629–34. doi:10.1086/322996. PMC 1235491. PMID 11462174.
Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H (Oct 2001). "DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor". The Journal of Biological Chemistry. 276 (40): 37556–63. doi:10.1074/jbc.M101730200. PMID 11477070.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (Jan 2003). "Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism". Science. 299 (5604): 256–9. doi:10.1126/science.1077209. PMID 12446870.
Bonifati V, Dekker MC, Vanacore N, Fabbrini G, Squitieri F, Marconi R, Antonini A, Brustenghi P, Dalla Libera A, De Mari M, Stocchi F, Montagna P, Gallai V, Rizzu P, van Swieten JC, Oostra B, van Duijn CM, Meco G, Heutink P (Sep 2002). "Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7". Neurological Sciences. 23 Suppl 2: S59–60. doi:10.1007/s100720200069. PMID 12548343.
Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SM, Ariga H (Feb 2003). "DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex". Molecular Cancer Research. 1 (4): 247–61. PMID 12612053.
Tao X, Tong L (Aug 2003). "Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease". The Journal of Biological Chemistry. 278 (33): 31372–9. doi:10.1074/jbc.M304221200. PMID 12761214.
Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H, Inagaki F (Aug 2003). "The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease". The Journal of Biological Chemistry. 278 (33): 31380–4. doi:10.1074/jbc.M305878200. PMID 12796482.
Dekker M, Bonifati V, van Swieten J, Leenders N, Galjaard RJ, Snijders P, Horstink M, Heutink P, Oostra B, van Duijn C (Jul 2003). "Clinical features and neuroimaging of PARK7-linked parkinsonism". Movement Disorders. 18 (7): 751–7. doi:10.1002/mds.10422. PMID 12815653.
Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu PP, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR (Sep 2003). "L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin-proteasome system". The Journal of Biological Chemistry. 278 (38): 36588–95. doi:10.1074/jbc.M304272200. PMID 12851414.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
Genes on human chromosome 1Uncategorized